
Cancer is losing its scare: thanks in part to diagnostics
The chances of surviving a cancer diagnosis have increased significantly in recent years. In addition to new, more targeted forms of treatment, advances in diagnostics are the main reason for this. Experienced oncologist Pavel Pisa is convinced that their importance will continue to grow. Diagnostics are essential for personalized diagnosis, but also for monitoring treatment and detecting resistance.
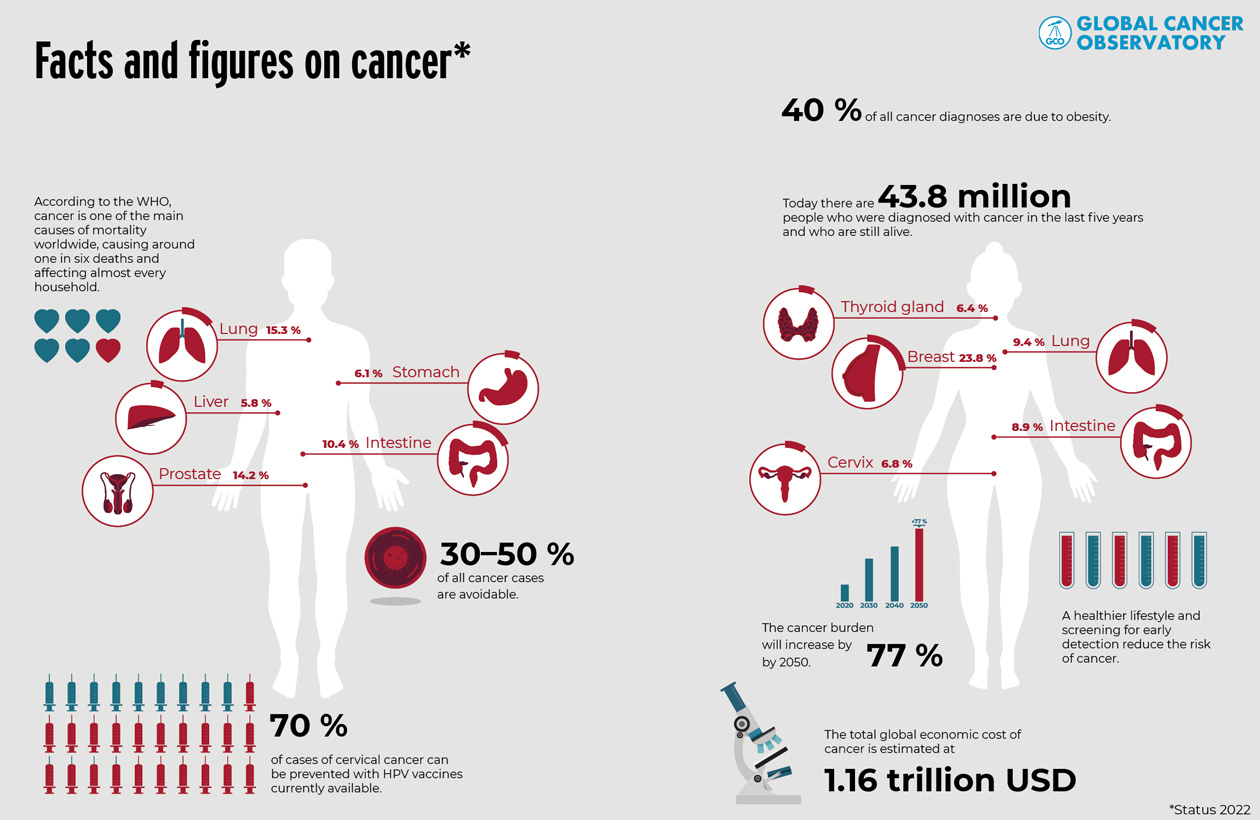
Cancer is the second leading cause of death worldwide, after cardiovascular disease. According to the World Health Organization (WHO), around 9 million people die each year from malignant tumors. But recent developments are encouraging. Mortality rates have fallen significantly over the past 20 years. In the US, for example, it has decreased by around 25 %.
Huge progress in the last 20 years
Especially in countries with a well-developed healthcare system, the diagnosis of cancer is becoming less frightening, confirms Pavel Pisa. A former professor of experimental clinical oncology at the Karolinska Institute in Sweden and former head of translational medicine at Basel-based pharmaceutical giant Roche, Pisa has more than 40 years’ experience in cancer research. “When I started as oncologist, my daily life was filled with frustration: patients first appeared with advanced stage disease, the available ’one fits all’ therapies were excessively harming the healthy tissues, and relapse, which always happened, was a death sentence,” he says.
Thanks to groundbreaking advancements in early detection, personalized therapies, and continuous monitoring, cancer is now becoming less of a feared diagnosis. Pisa is optimistic: “There are still many challenges, but compared to the 1990s, the situation today is very different. Curing cancer is no longer a pipe dream. ’It is not even the beginning of the end. But it is, perhaps, the end of the beginning,’ to quote Winston Churchill.”
Genomic diagnostics pave the way for personalized medicine
According to Pisa, the main reason for the great progress made in recent years lies in diagnostic techniques. On the one hand, they make it possible to detect tumors at an increasingly early stage and thus combat them more effectively. On the other hand, NGS has made it possible to pinpoint the genetic changes in cells and gradually understand them better.
The findings of NGS are changing the face of oncology, as Pisa points out: “Just imagine that with a lung X-ray you can see lumps of the size of 2–4 cm and with a CT scan you can focus down to 1 cm. That area corresponds to 100 million tumor cells. With molecular diagnostics we can detect circulating tumor DNA at concentrations below 0.1 % of all cellfree DNA found in blood samples. It is not only the sensitivity we see, but also the information we obtain nowadays that is mindboggling. We have so many subgroups of a tumor type that we previously called by just one name: ’lung or breast cancer’! Now, we do not speak about ’targeted’ but ’personalized’ therapies, tailored to the genetic makeup of an individual tumor in the patient.”
For pharmaceutical companies, the personalization of therapies also means moving away from blockbuster drugs. For the diagnostic industry it creates greater volumes and workload, increased through-put and higher speed.
For the clinical oncologist it requires managing and correctly interpreting such information and constantly following new advances in the field. For the patient, as the statistics prove, it means not only “life extension” but also higher chances of combating cancer death with maintained quality of life.
Personalized therapies are transforming cancer from a death sentence into a manageable condition.
Successful drug- and cell-based immunotherapies
Targeted immunotherapies are among the treatments that have been successfully personalized in recent years.
These include drugs such as checkpoint inhibitors, which block specific receptors that a tumor uses to downregulate the immune system, or tumor-specific antibodies produced in the laboratory.
The latest patient-specific immunotherapy, which can now be used to fight metastases throughout the body, is chimeric antigen receptor (CAR) T-cell therapy. T lymphocytes, which are part of the patient’s white blood cells, are manipulated in the laboratory to act as “drug cells” and attack a specific surface antigen on the individual tumor. In many patients, this not only significantly reduces the tumor burden throughout the body. In some cases, it is even possible to clear the body of malignant cancer cells completely.
Mandatory automation requires understanding of the application
As successful as CAR T-cell therapy has been in clinical practice, the cost barriers to its widespread use are just as great. The production of individual T cells alone costs more than CHF 100,000.
Today, it is still largely based on manual work that has to be carried out by specialists in sophisticated “germfree” facilities under difficult labor conditions. A lot of resources could be saved by automating the processes and thus making it more accessible to a wider patient population.
However, automating such specific medical therapies is extremely challenging, as Pisa knows from experience: “It requires engineers who not only know the technological side of the device. They also need to understand the biology and the laboratory reality of the application.

This is the only way they can discuss with manufacturers on an equal footing and find a solution that can effectively reduce costs and improve the real-life manufacturing conditions. It needs to be a joint effort, not the world’s best solution to an issue that doesn’t exist.
Conversely, sometimes the biologists don’t even know about technical solutions to mitigate their workload.”
“I have seen many promising innovations from scientists that have not been turned into a marketable product. And often the reason was that the development partner did not properly understand the biology behind the process and the specific market conditions in the life sciences,” says Pisa.
Small molecules with a big future
According to Pisa, medicine has by no means reached the end of the road with targeted immunotherapies such as CAR T-cell: “Currently, so-called antibody-drug conjugates, in which tumor-specific antibodies are combined with cell-destroying agents, are on their way into clinical practice. For example, chemotherapeutic agents or radioactive isotopes can be targeted to the tumor cells. By targeting the tumor instead of the entire body, they are much more effective and have far fewer side effects.”
Pisa also sees a great future for targeted small-molecule drugs. They are designed to bind specifically to certain tumor proteins and block the division of cancer cells. They could also be used instead of antibodies for the targeted transport of cell-destroying substances.
Diagnostics crucial during and after treatment
Regardless of which form of therapy is most successful in curing cancer in the future, diagnostics will play an increasingly important role in all of them. As Pisa explains, diagnostics are no longer just crucial for the precise identification of tumors before personalized therapy: “Cancer is not a disease that can be detected and then cured forever with a single treatment. Tumors evolve. With each cell division, new mutations occur and, over time, resistance to an initially successful therapy can develop.”
But today we can also respond much better to these changes in the tumor, states Pisa: “Formerly we had only one line of treatment to offer to a patient and the majority of our patients were too sick to receive any treatments when the cancer came back. Now there are three to four treatment lines that patients are fit to receive later, depending on how their cancer changes.”
As the correct treatment choice depends on diagnostics, pharmaceutical companies today often develop diagnostic tools in parallel with a new therapy submission. With systematic monitoring, relapses can be detected early and new targets for the treatment of the mutated cancer can be systematically identified.
Science, clinics, pharmaceuticals and engineering together
Pisa’s many years of experience have shown that the key to translating new scientific findings into successful therapies is the best possible interaction between all the players involved: from scientific research at universities, through translation into pharmaceutical therapeutics by industry and clinical practice in university hospitals, to the engineering of automation solutions that enable widespread and reliable application.
According to Pisa, Switzerland is particularly well positioned in this respect. It has worldclass companies and research organizations in all key areas, concentrated in a very small geographical area. “In Switzerland, I can find first-class specialists in practically every field of expertise, and they can even meet in person quickly and easily if necessary. This makes interdisciplinary collaboration even easier.”
Sources:
pharma-fakten.de
www.who.int
cancerstatisticscenter.cancer.org
www.bfs.admin.ch

About Pavel Pisa, M.D., PhD.
Pavel Pisa has extensive Translational Medicine experience gained at world leading academic medical institutions and working at Roche Pharmaceuticals in Switzerland.
His scientific expertise in tumor immunology and cancer immunotherapy is documented in 120 peer-reviewed and highly cited publications. Pavel has held positions as professor and clinical head of oncology, hematology and internal medicine at the Karolinska Institute, Sweden.
During his time at Roche, Pavel was at leading positions and a key contributor to multiple successful clinical development programs in cancer immunotherapy. His work generated multiple patents and awards, e.g. “The Drug Discovery of the Year Award 2015” by the British Pharmacological Society.

Recent Annual Reports



